Embark on a scientific odyssey with the heating curve worksheet 1 answer key, your trusted guide to deciphering the intricate dance of temperature and energy. This comprehensive resource unlocks the mysteries of heating curves, empowering you to analyze thermal transitions and delve into the fascinating world of material characterization.
Prepare to unravel the secrets of heating curves as we explore their significance, interpret their graphs, and uncover their applications in diverse fields. Get ready to witness the transformation of solids into liquids and gases, unravel the mysteries of specific heat capacity and enthalpy, and delve into the practical implications of heating curve analysis.
Worksheet Overview
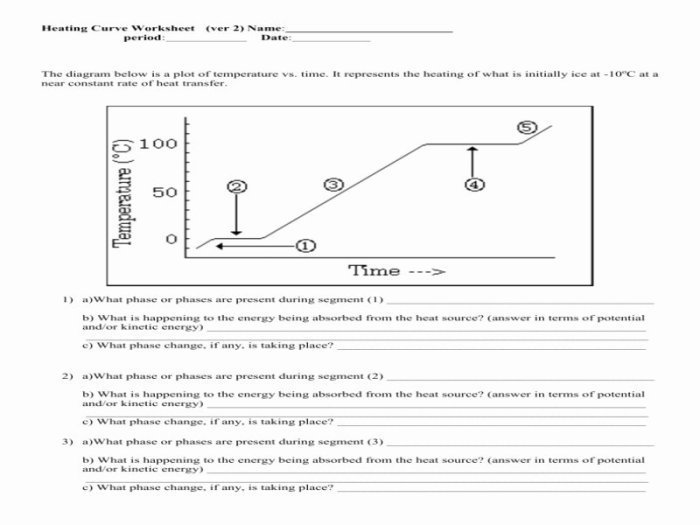
A heating curve worksheet is a valuable tool used to analyze the thermal behavior of a substance as it undergoes a change in temperature.
Typically, a heating curve worksheet consists of the following components:
Data Table
- Temperature: This column records the temperature of the substance at regular intervals.
- Heat Flow: This column measures the amount of heat absorbed or released by the substance during the temperature change.
Graph
The data from the table is plotted on a graph, with temperature on the x-axis and heat flow on the y-axis. This graph provides a visual representation of the substance’s thermal behavior.
Analysis
The graph is analyzed to identify the different stages of the heating process, such as melting, boiling, and freezing. By examining the shape of the curve, scientists can determine the specific heat capacity, latent heat of fusion, and latent heat of vaporization of the substance.
Heating Curve Concepts
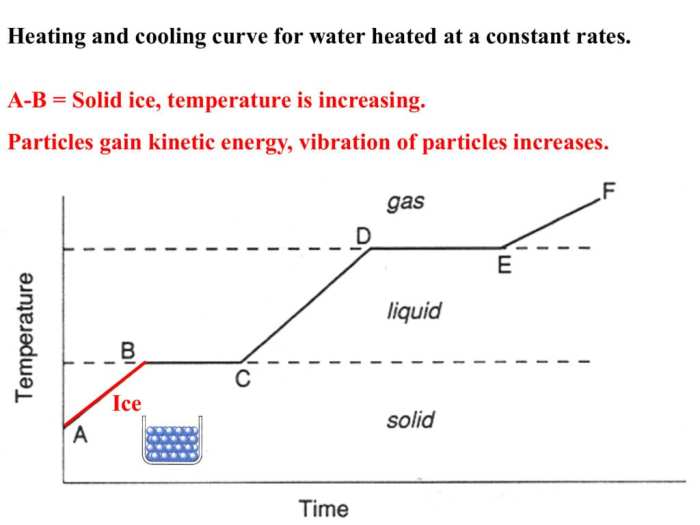
A heating curve is a graphical representation of the temperature change of a substance as heat is added to it at a constant rate. It is a useful tool for understanding the physical changes that occur in a substance as it is heated.
The heating curve of a substance typically consists of several stages, each of which corresponds to a different physical change. The stages of a heating curve are as follows:
Solid Stage
- In the solid stage, the substance is in a solid state and its temperature increases linearly as heat is added.
- The slope of the heating curve in the solid stage is equal to the specific heat capacity of the substance.
Melting Stage
- At the melting point, the substance begins to melt. The temperature remains constant during melting as the heat is used to overcome the intermolecular forces holding the molecules in place.
- The melting point is a characteristic property of a substance and is dependent on the pressure.
Liquid Stage
- In the liquid stage, the substance is in a liquid state and its temperature increases linearly as heat is added.
- The slope of the heating curve in the liquid stage is equal to the specific heat capacity of the liquid.
Boiling Stage
- At the boiling point, the substance begins to boil. The temperature remains constant during boiling as the heat is used to overcome the intermolecular forces holding the molecules together and convert them into a gas.
- The boiling point is a characteristic property of a substance and is dependent on the pressure.
Gas Stage
- In the gas stage, the substance is in a gas state and its temperature increases linearly as heat is added.
- The slope of the heating curve in the gas stage is equal to the specific heat capacity of the gas.
Relationship Between Temperature and Energy During Heating
The relationship between temperature and energy during heating is that the temperature of a substance increases as heat is added to it. This is because the heat energy causes the molecules of the substance to move faster, which in turn increases the temperature.
The amount of heat required to raise the temperature of a substance by 1 degree Celsius is known as the specific heat capacity of the substance. The specific heat capacity is a characteristic property of a substance and is dependent on the substance’s chemical composition and physical state.
Data Analysis
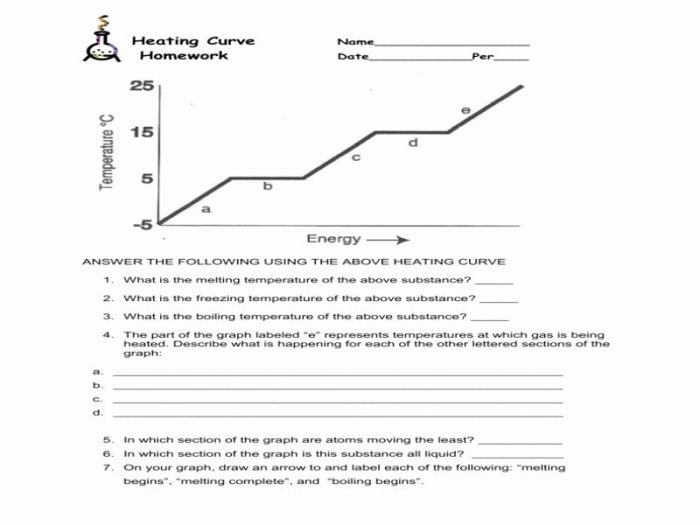
The heating curve graph is a valuable tool for understanding the thermal behavior of a substance as it undergoes physical changes. By analyzing the graph, we can identify key points, such as the melting point and boiling point, and calculate important thermodynamic properties, such as specific heat capacity and enthalpy of fusion/vaporization.
To interpret a heating curve graph, it is important to understand the different regions of the graph. The initial region, where the temperature rises steadily, represents the solid phase. The melting point is the temperature at which the solid begins to melt, and it is indicated by a plateau in the graph.
During melting, the temperature remains constant as the substance absorbs energy to overcome the intermolecular forces holding the solid together. Once the substance is completely melted, the temperature begins to rise again, indicating the liquid phase. The boiling point is the temperature at which the liquid begins to boil, and it is indicated by another plateau in the graph.
During boiling, the temperature remains constant as the substance absorbs energy to overcome the intermolecular forces holding the liquid together and transform into a gas.
Calculating Specific Heat Capacity
The specific heat capacity of a substance is the amount of heat required to raise the temperature of one gram of the substance by one degree Celsius. It can be calculated using the following formula:
Cp= Q / (m
ΔT)
where:
- C pis the specific heat capacity (J/g°C)
- Q is the heat added to the substance (J)
- m is the mass of the substance (g)
- ΔT is the change in temperature (°C)
To calculate the specific heat capacity from a heating curve graph, we need to identify the region of the graph where the substance is in the solid or liquid phase and the temperature is rising steadily. We then measure the slope of the line in that region, which is equal to Q/(m – ΔT).
The specific heat capacity is then equal to the slope of the line.
Calculating Enthalpy of Fusion/Vaporization
The enthalpy of fusion is the amount of heat required to melt one mole of a substance, and the enthalpy of vaporization is the amount of heat required to vaporize one mole of a substance. Both can be calculated using the following formula:
ΔH = Q / n
where:
- ΔH is the enthalpy of fusion or vaporization (J/mol)
- Q is the heat added to the substance (J)
- n is the number of moles of the substance (mol)
To calculate the enthalpy of fusion or vaporization from a heating curve graph, we need to identify the plateau in the graph where the substance is melting or boiling. We then measure the area under the plateau, which is equal to Q.
The enthalpy of fusion or vaporization is then equal to the area under the plateau divided by the number of moles of the substance.
Applications and Examples: Heating Curve Worksheet 1 Answer Key
Heating curve analysis is a powerful tool used in various scientific and industrial fields to characterize materials and optimize thermal processes.
By analyzing the temperature-dependent changes in a substance’s properties, heating curves provide insights into its physical and chemical behavior.
Material Characterization, Heating curve worksheet 1 answer key
- Phase Transitions:Heating curves can identify phase transitions, such as melting, freezing, and boiling, by detecting changes in the substance’s specific heat capacity.
- Thermal Properties:The shape and slope of a heating curve can reveal thermal properties, including specific heat capacity, enthalpy of fusion, and enthalpy of vaporization.
- Composition Analysis:Heating curves can be used to differentiate between materials with similar compositions by comparing their characteristic temperature changes during phase transitions.
Thermal Processes
- Process Optimization:Heating curves aid in optimizing thermal processes, such as annealing, sintering, and crystallization, by determining the appropriate temperatures and heating rates.
- Material Selection:Heating curves assist in selecting materials with specific thermal properties for various applications, such as high-temperature resistance or thermal stability.
- Quality Control:Heating curves can be used for quality control purposes to ensure the consistency and integrity of materials and products.
HTML Table for Data Presentation
An HTML table provides a structured and organized way to present heating curve data. It allows for easy comparison and analysis of the data points.
To create an HTML table, use the
| tag. The table headers are specified using the | tag.
Formatting the Table
Example HTML TableThe following is an example HTML table with sample heating curve data:
Blockquote for Procedure
Blockquotes provide a structured way to present step-by-step procedures, emphasizing key safety precautions and ensuring clarity in conducting experiments. They enhance the readability and comprehension of experimental protocols. The following blockquote Artikels a detailed procedure for conducting a heating curve experiment: Procedure
Key Questions AnsweredWhat is the purpose of a heating curve worksheet? A heating curve worksheet provides a structured framework for recording and analyzing temperature changes during a heating process, enabling the identification of key thermal transitions and the calculation of important thermodynamic properties. How do I interpret a heating curve graph? Heating curve graphs depict the relationship between temperature and time or energy. By analyzing the shape and features of the curve, you can identify phase transitions (e.g., melting, boiling), calculate specific heat capacity, and determine the enthalpy of fusion or vaporization. What are the applications of heating curve analysis? Heating curve analysis finds widespread applications in material characterization, thermal process optimization, and quality control. It helps identify unknown materials, determine purity, study phase transitions, and optimize heating and cooling cycles in industrial processes. |
|---|