Draw the structure of 1 2 epoxypentane – Draw the structure of 1,2-epoxypentane and delve into the fascinating realm of organic chemistry. This epoxide compound, with its unique structure and properties, plays a crucial role in various industrial applications. Join us as we explore its intricacies, from its IUPAC nomenclature to its synthesis and practical uses.
In this comprehensive guide, we will provide a detailed overview of 1,2-epoxypentane, including its structural formula, physical and chemical properties, methods of synthesis, and industrial applications. Additionally, we will discuss safety precautions and handling guidelines to ensure responsible use of this versatile compound.
1,2-Epoxypentane
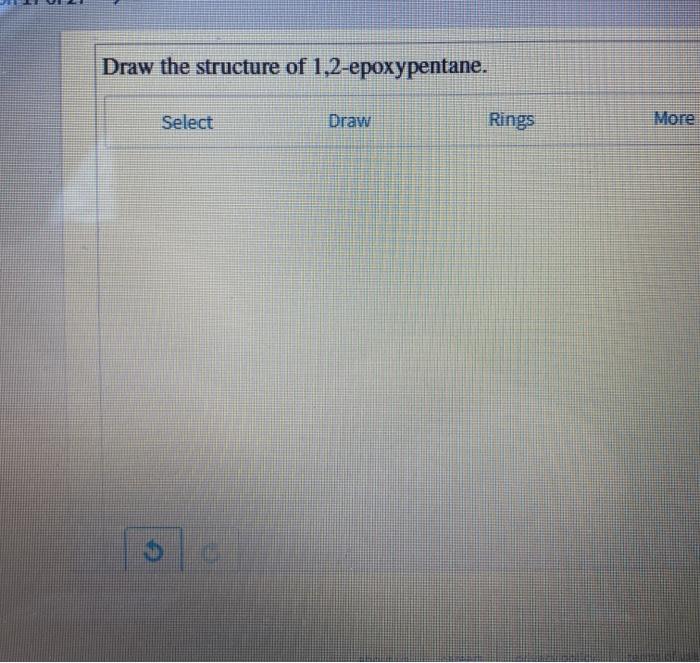
,2-epoxypentane is an organic compound with the molecular formula C5H10O. It is a colorless liquid with a characteristic odor. The molecule consists of a five-carbon chain with an epoxide functional group on the first and second carbon atoms. The epoxide functional group is a three-membered ring consisting of two carbon atoms and one oxygen atom.
IUPAC Nomenclature
The IUPAC name for 1,2-epoxypentane is oxirane. The prefix “oxirane” indicates the presence of the epoxide functional group. The suffix “-ane” indicates that the compound is an alkane. The numbers “1,2” indicate the location of the epoxide functional group on the carbon chain.
Physical and Chemical Properties
,2-epoxypentane is a colorless liquid with a boiling point of 101 °C and a density of 0.83 g/mL. It is soluble in water and organic solvents. The epoxide functional group is a reactive functional group that can undergo a variety of reactions, including ring-opening reactions, addition reactions, and polymerization reactions.
Synthesis and Applications
,2-epoxypentane can be synthesized by the reaction of pentene with peroxyacids. It is also produced as a byproduct of the epoxidation of pentene. 1,2-epoxypentane is used as a reactive intermediate in the synthesis of other organic compounds. It is also used as a solvent and as a plasticizer.
Safety and Handling, Draw the structure of 1 2 epoxypentane
,2-epoxypentane is a flammable liquid that can cause skin and eye irritation. It is also a suspected carcinogen. 1,2-epoxypentane should be handled with care and proper protective equipment should be worn.
Essential Questionnaire: Draw The Structure Of 1 2 Epoxypentane
What is the IUPAC name for 1,2-epoxypentane?
The IUPAC name for 1,2-epoxypentane is oxirane,2-pentyl-.
What are the physical properties of 1,2-epoxypentane?
1,2-Epoxypentane is a colorless liquid with a boiling point of 156-157 °C, a density of 0.86 g/mL, and a refractive index of 1.432.
What are the chemical reactions that 1,2-epoxypentane can undergo?
1,2-Epoxypentane can undergo a variety of reactions, including nucleophilic addition, electrophilic addition, and rearrangement reactions.