History of the atom webquest – Prepare to unravel the captivating history of the atom in this engaging webquest. Join us on an adventure that traces the evolution of our understanding of the atom, from its humble beginnings in ancient Greece to the groundbreaking discoveries of the modern era.
Through interactive timelines, captivating visuals, and thought-provoking discussions, we’ll delve into the minds of brilliant scientists who shaped our knowledge of the atom, unraveling the mysteries of its structure and unlocking its immense potential.
Historical Timeline of the Atom: History Of The Atom Webquest
The concept of the atom has evolved over centuries, from ancient philosophical ideas to the sophisticated models we use today. This timeline highlights key milestones in our understanding of the atom.
The ancient Greek philosopher Democritus first proposed the idea of indivisible particles called atoms around 400 B.C. However, this idea was largely ignored until the 17th century.
John Dalton’s Atomic Theory
- In 1803, John Dalton proposed his atomic theory, which stated that all matter is composed of tiny, indivisible particles called atoms.
- He also suggested that atoms of the same element are identical in mass and properties.
- Dalton’s theory was a significant step forward in understanding the atom, but it had some limitations.
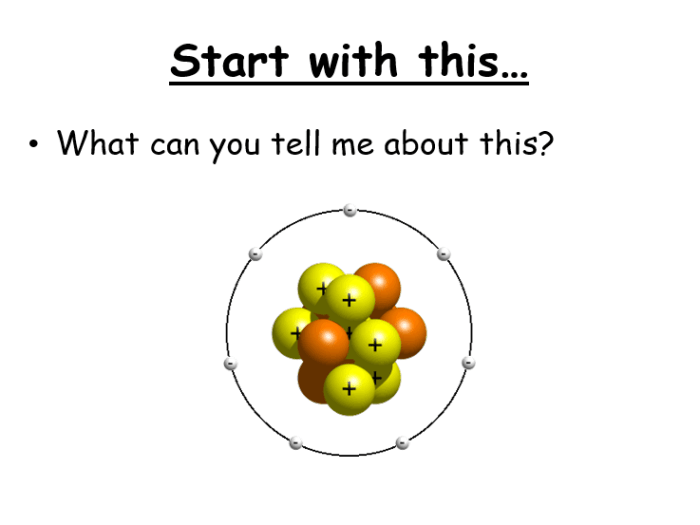
Structure of the Atom
An atom is the basic unit of matter and the smallest unit of an element that can exist independently. It consists of a central nucleus and electrons that orbit around it.
Subatomic Particles
Atoms are composed of three subatomic particles: protons, neutrons, and electrons.
- Protonsare positively charged particles located in the nucleus.
- Neutronsare neutral particles located in the nucleus.
- Electronsare negatively charged particles that orbit the nucleus in specific energy levels.
Atomic Number, Mass Number, and Isotopes
The atomic numberof an atom is the number of protons in its nucleus, which determines the element to which it belongs.
The mass numberof an atom is the sum of the number of protons and neutrons in its nucleus.
Isotopesare atoms of the same element that have the same atomic number but different mass numbers. This is due to variations in the number of neutrons in the nucleus.
Properties of Subatomic Particles
| Particle | Charge | Location |
|---|---|---|
| Proton | +1 | Nucleus |
| Neutron | 0 | Nucleus |
| Electron | -1 | Orbits the nucleus |
Atomic Models
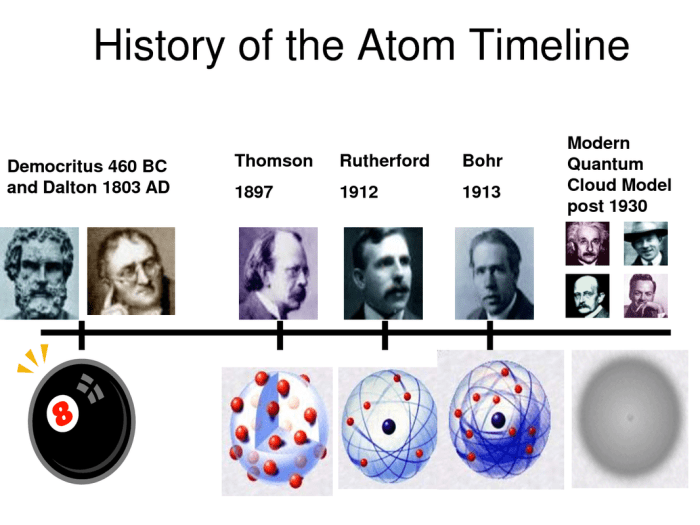
The evolution of atomic models has been a continuous journey, with each new model refining our understanding of the structure and behavior of atoms. Here, we’ll compare and contrast the major atomic models and discuss their strengths and limitations.
Dalton’s Model
Proposed by John Dalton in 1803, Dalton’s model was the first to propose that matter is composed of indivisible, spherical atoms. It successfully explained the law of conservation of mass and the law of definite proportions. However, it failed to account for the structure of atoms and the existence of subatomic particles.
Bohr’s Model
Niels Bohr’s model, proposed in 1913, introduced the concept of energy levels and electron orbits. It explained the emission and absorption spectra of atoms and provided a framework for understanding atomic structure. However, it could not explain the behavior of electrons in atoms with more than one electron.
Quantum Mechanical Model
The quantum mechanical model, developed in the 1920s, is the most accurate and comprehensive model of the atom. It incorporates the principles of quantum mechanics and provides a probabilistic description of electron behavior. This model explains the complex behavior of electrons in atoms and forms the basis of modern chemistry and physics.
Quantum Mechanics and the Atom
Quantum mechanics is a branch of physics that deals with the behavior of matter at the atomic and subatomic level. It provides a more accurate and complete understanding of the atom than classical physics.Quantum mechanics introduces several fundamental principles that challenge our classical intuition.
One of the key principles is wave-particle duality, which states that particles such as electrons can also behave like waves. This means that electrons can exhibit properties of both particles (such as mass and charge) and waves (such as interference and diffraction).Another
important concept in quantum mechanics is energy levels. According to quantum theory, electrons in an atom can only occupy specific discrete energy levels. These energy levels are determined by the size and shape of the atom’s orbitals. Orbitals are three-dimensional regions around the nucleus where electrons are most likely to be found.Quantum
mechanics has revolutionized our understanding of the atom. It has provided a more accurate and complete description of the atom’s structure and behavior. Quantum mechanics has also led to the development of new technologies, such as lasers and transistors, which are essential for modern society.
Wave-Particle Duality
The wave-particle duality of electrons is a fundamental property of matter at the quantum level. It means that electrons can behave like both particles and waves, depending on the experimental setup.For example, in the double-slit experiment, electrons passing through two closely spaced slits create an interference pattern on a screen behind the slits.
This pattern is characteristic of waves and cannot be explained by classical physics.However, in other experiments, electrons exhibit particle-like behavior. For example, they can be detected as individual particles with a specific position and momentum.The wave-particle duality of electrons is one of the most counterintuitive aspects of quantum mechanics.
It challenges our classical intuition about the nature of matter.
Energy Levels, History of the atom webquest
According to quantum theory, electrons in an atom can only occupy specific discrete energy levels. These energy levels are determined by the size and shape of the atom’s orbitals. Orbitals are three-dimensional regions around the nucleus where electrons are most likely to be found.The
Have you ever wondered what’s in store for you? From the history of the atom webquest to qué venden en la tienda , there’s always something new to discover. So dive into the world of knowledge and uncover the hidden treasures that await you in the vast expanse of the atom.
lowest energy level is the ground state. As electrons gain energy, they move to higher energy levels. The highest energy level that an electron can occupy is called the excited state.Electrons can move between energy levels by absorbing or emitting photons of light.
The wavelength of the photon is determined by the energy difference between the two energy levels.The energy levels of electrons are important because they determine the atom’s chemical properties. For example, the number of electrons in the outermost energy level determines the atom’s valence, which affects its ability to bond with other atoms.
Orbitals
Orbitals are three-dimensional regions around the nucleus where electrons are most likely to be found. Orbitals are defined by their shape, energy, and orientation.There are four types of orbitals: s, p, d, and f. The s orbitals are spherical in shape.
The p orbitals are dumbbell-shaped. The d orbitals have four lobes, and the f orbitals have eight lobes.Each orbital can hold a maximum of two electrons. The electrons in an orbital must have opposite spins.The shape of an orbital is determined by the quantum numbers of the electron that occupies it.
The quantum numbers are:* Principal quantum number (n): This number describes the size and energy of the orbital.
Azimuthal quantum number (l)
This number describes the shape of the orbital.
Magnetic quantum number (ml)
This number describes the orientation of the orbital.
Spin quantum number (ms)
This number describes the spin of the electron.Orbitals are important because they determine the chemical properties of atoms. For example, the number of electrons in the outermost orbital determines the atom’s valence, which affects its ability to bond with other atoms.
Applications of Atomic Science
Atomic science has revolutionized numerous fields, including medicine, energy, and materials science. Its practical applications have greatly enhanced our technological capabilities and improved our quality of life.
Harnessing Atomic Energy
Atomic energy, derived from nuclear reactions, has been harnessed for power generation and medical treatments. Nuclear power plants use controlled nuclear reactions to produce electricity, providing a clean and efficient energy source.
In medicine, radiation therapy utilizes atomic energy to target and destroy cancerous cells, offering a precise and effective treatment option. Radioisotopes, produced through nuclear reactions, are used in medical imaging techniques such as PET and MRI, allowing for accurate diagnosis and monitoring of various diseases.
Advanced Materials
Atomic science has led to the development of advanced materials with exceptional properties. These materials find applications in various industries, including aerospace, electronics, and healthcare.
- Nanomaterials:Extremely small materials with unique properties, such as increased strength and reactivity, are used in sensors, drug delivery systems, and electronic devices.
- Superconductors:Materials that conduct electricity without resistance, enabling efficient energy transmission and the development of powerful magnets.
- Composite materials:Combinations of different materials with tailored properties, providing enhanced strength, durability, and lightweight properties.
FAQ Compilation
What is the significance of the discovery of the atom?
The discovery of the atom laid the foundation for our understanding of the physical world. It revealed that all matter is composed of tiny, indivisible units, providing a framework for explaining the properties and behavior of substances.
How has atomic science impacted modern technology?
Atomic science has revolutionized various fields, including medicine, energy, and materials science. It has led to advancements such as nuclear power, medical imaging techniques, and the development of new materials with enhanced properties.
What are the key differences between Dalton’s atomic model and the quantum mechanical model?
Dalton’s atomic model proposed that atoms are indivisible, solid spheres, while the quantum mechanical model describes atoms as complex systems with a nucleus surrounded by a cloud of electrons. The quantum mechanical model incorporates wave-particle duality and energy level quantization, providing a more accurate representation of atomic behavior.
